PARP1 and PARP2 are dispensable for DNA repair by microhomology-mediated end-joining during mitosis.
PARP1 and PARP2 are dispensable for DNA repair by microhomology-mediated end-joining during mitosis.
Ortega, R.; Taylor, E.; Whitehead, S. M.; Danhorn, T.; Bitler, B. G.; Arnoult, N. G.
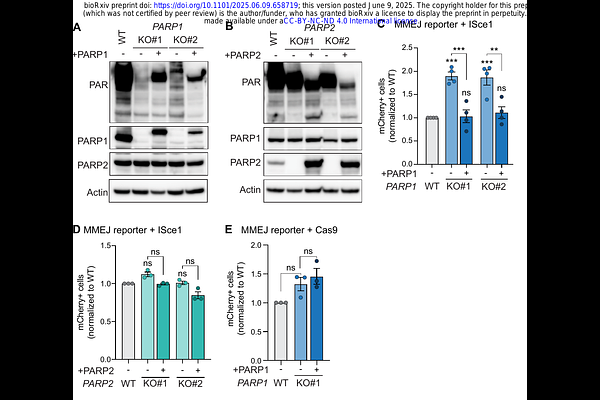
AbstractPoly ADP-ribose polymerase (PARP) inhibitors are standard of care treatment for cancers with homologous-recombination deficiencies. Yet, as tumors develop resistance, complementary strategies are emerging, including targeting microhomology-mediated end joining (MMEJ). Given that PARP1 is widely described as a key promoter of MMEJ, one would expect PARP inhibition to suppress MMEJ, potentially making MMEJ inhibition redundant. MMEJ was originally described as a backup pathway, with seminal work linking PARP1 to MMEJ conducted in NHEJ-deficient cells, where MMEJ can be reactivated in G1. However, we now appreciate that MMEJ is a mitotic repair mechanism, but the function of PARP1 in this context remains unclear. Here, we systematically explore the effect that PARP/PARPi have on MMEJ activity in cells with intact NHEJ. Surprisingly, PARP inhibition leads to elevated PolQ-dependent MMEJ levels at ISceI-mediated DSBs, which we find is due to PARP1 regulation of competing pathways at these breaks. Importantly, we show that PARP is dispensable for MMEJ at double-ended DSBs and is expendable for repair of DSBs during mitosis. Altogether, this data shifts the understanding of PARP role in MMEJ and DNA repair pathway choice. Moreover, we believe this data further strengthens a rationale for PARPi/MMEJi combinatorial drug treatment in HR-deficient cancers.