TRiC-assisted folding of class I HDAC family proteins regulated by distinct co-chaperone and cofactor networks
TRiC-assisted folding of class I HDAC family proteins regulated by distinct co-chaperone and cofactor networks
li, z.; Zhao, Q.; jiang, w.; song, q.; zhou, x.; shi, x.; zhang, q.; wang, y.; lin, y.; Yin, Y.; pan, c.; Cong, Y.
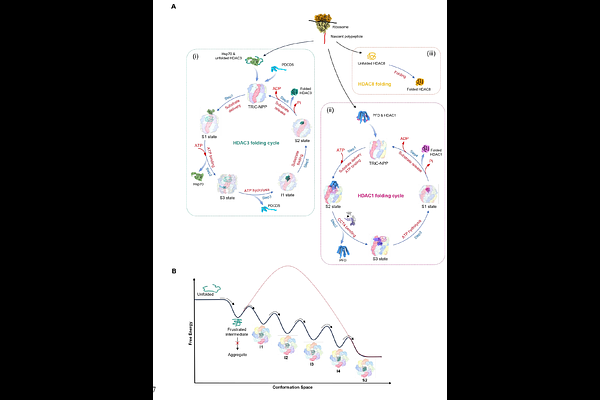
AbstractClass I histone deacetylases (HDACs), including HDAC1, HDAC2, HDAC3, and HDAC8, are essential for diverse cellular processes. Although the chaperonin TRiC is implicated in the activation of class I HDACs, the underlying mechanisms remain elusive. Using cryo-electron microscopy (cryo-EM), cross-linking mass spectrometry (XL-MS), and biochemistry analyses, we established class I HDACs as novel TRiC substrates and elucidate TRiC-assisted folding of HDAC1 and HDAC3 during its ATPase cycle, orchestrated by distinct co-chaperone and cofactor networks. In the closed TRiC chamber, both HDAC1 and HDAC3 adopt near-native states with shared binding interfaces. However, their open-state configurations diverge: Hsp70 and PDCD5 engage atop and within TRiC, respectively, for HDAC3, whereas prefoldin (PFD) binds atop TRiC for HDAC1, suggesting roles in substrate delivery and folding modulation. Furthermore, an unexpected bent conformation of CCT4, detected in TRiC-HDAC1 complex, may facilitate co-chaperone dissociation from TRiC. In contrast, HDAC8 folds independently of TRiC. Our study reveals the mechanism governing TRiC-assisted folding of class I HDACs in orchestration of dynamic co-chaperone/cofactor network, shielding new lights on the sophisticated regulatory landscape of TRiC, and open promising avenues for designing peptides or small molecules to selectively modulate TRiC-assisted folding of class I HDACs and other substrates.