Cytoplasmic tail composition modulates the G protein and arrestin-3 signaling bias of the adhesion GPCR LPHN2
Cytoplasmic tail composition modulates the G protein and arrestin-3 signaling bias of the adhesion GPCR LPHN2
Garbett, K.; Zheng, C.; Drube, J.; Hoffmann, C.; Gurevich, V.; Sando, R.
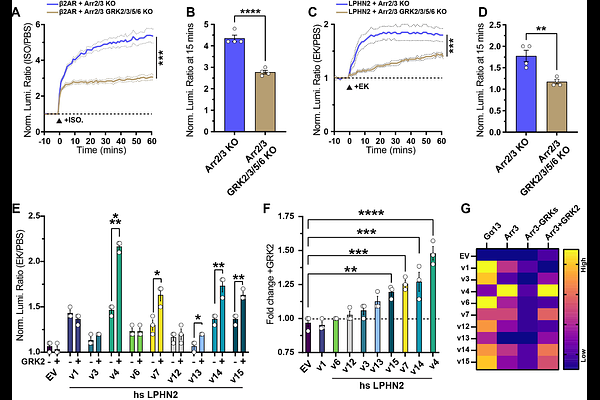
AbstractThe class B2 Adhesion GPCRs (aGPCRs) combine cell adhesion with GPCR signaling to control diverse developmental and physiological processes. How aGPCRs interact with and integrate distinct groups of effectors including G proteins, arrestins, and G protein receptor kinases (GRKs) remains unclear. Here, we find that diversity in the aGPCR C-terminal intracellular tail modulates G protein activation, arrestin-3 recruitment, and utilization of selective GRKs in LPHN2, a postsynaptic aGPCR essential for synapse formation. The C-terminal tail of LPHN2 is required for G protein activation and arrestin-3 recruitment. LPHN2 with an intact tail recruits arrestin-3 in the absence of G protein activation, suggesting constitutive arrestin-3-biased signaling. Alternative splicing of the LPHN2 tail modulates G protein activation and arrestin-3 binding independently, supporting that it controls G protein vs arrestin-3 bias. GRKs are important but not essential for arrestin-3 recruitment to LPHN2. Moreover, GRK2 increases arrestin-3 recruitment only in a subset of LPHN2 variants. Collectively, these results show that the mechanisms of the interactions of class B2 aGPCRs and arrestin are distinct from those of class A GPCRs and that splicing of the LPHN2 C-terminal tail determines G protein vs arrestin bias.