Drosophila SA1 expression prevents brain tumorigenesis and PARP-mediated cell elimination
Drosophila SA1 expression prevents brain tumorigenesis and PARP-mediated cell elimination
Totaro, S.; Lettieri, A.; Castiglioni, S.; Lavezzari, F.; Gervasini, C.; Massa, V.; vaccari, t.
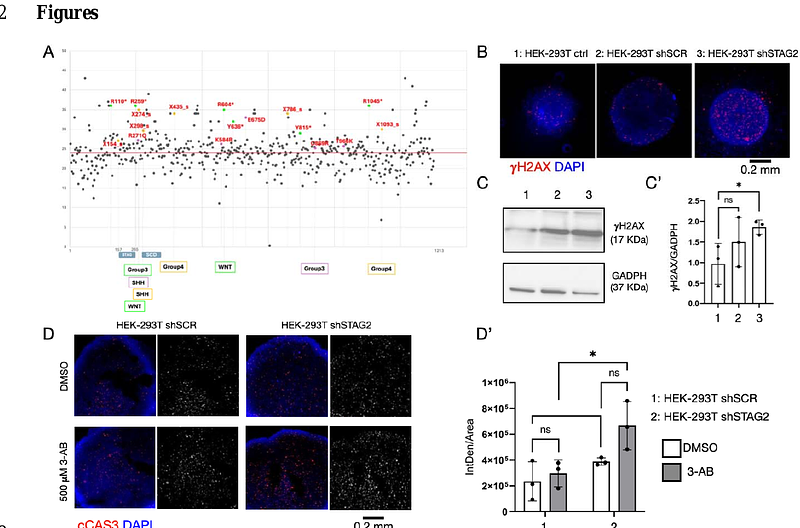
AbstractThe cohesin complex performs essential cellular functions including regulation of chromosome cohesion, chromatin organization and DNA repair. Somatic pathogenetic variants in cohesin genes, such as STAG2, have been associated with cancer, but their contribution to brain tumorigenesis is unclear. Here, we report the presence of STAG2 variants in glioblastoma and medulloblastoma patients and determine that loss of STAG2 in human cells leads to DNA damage and apoptosis. Treatment with inhibitors of the Poly ADP-ribose polymerase (PARP), which are used to treat forms of cancer with defects in DNA repair, increased the amount of apoptosis, confirming that synthetic lethality between reduced cohesin and PARP activity could be observed in vitro. Similar results were obtained in vivo by reducing expression of SA1, the Drosophila melanogaster homolog of STAG1/2. Cohesin gene silencing during fly brain development leads to defects in neural stem cells differentiation and tumorigenesis both in the presence of oncogenic activity and per se. Our in vivo and in vitro data suggests that impairment of PARP activity might induce synthetic lethality in cohesin-dependent tumors, highlighting a vulnerability that can be pharmacologically exploited.