Phosphorylation of a bacterial response regulator by noncognate kinase links carbon catabolite repression to phosphate starvation response
Phosphorylation of a bacterial response regulator by noncognate kinase links carbon catabolite repression to phosphate starvation response
Park, J.-Y.; Abdel-Fattah, W. R.
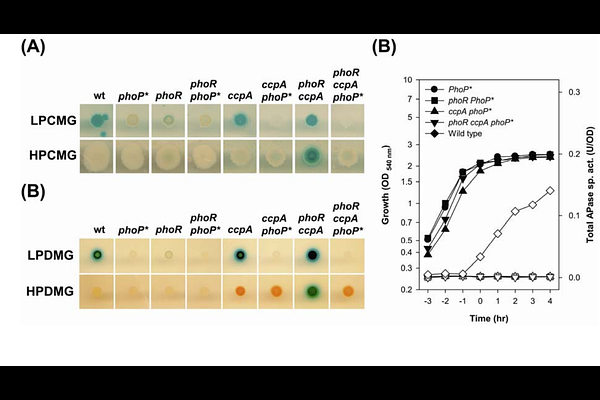
AbstractThe PhoP-PhoR two-component system regulates transcription of the Pho regulon to counteract inorganic phosphate (Pi) limitation. Interestingly, in a ccpA mutant lacking the global regulator of carbon catabolism, Pho regulon genes are hyper-induced in a PhoR-independent, glucose-dependent manner. While CcpA is known to directly repress the phoP promoter, we now show that overexpression of a phosphorylatable form of PhoP is essential for this hyper-induction. Remarkably, we detected early PhoP phosphorylation in the ccpA phoR double mutant. PhosTag-based analysis revealed that the phosphorylated/unphosphorylated PhoP ratio, rather than absolute levels, is the critical determinant of Pho regulon activation under these conditions. Given that PhoR can phosphorylate non-cognate YycF, the response regulator of the essential YycFG system controlling cell wall metabolism. We hypothesized that PhoP phosphorylation in the absence of PhoR may be mediated by YycG. Supporting this, PhoP co-immunoprecipitated with YycG, and in vitro phosphorylation assays confirmed direct phosphorylation of PhoP by YycG. We propose a Pi-stress regulatory model in which CcpA prevents excessive PhoP accumulation, thereby maintaining an appropriate balance between phosphorylated and unphosphorylated PhoP. This regulatory balance prevents aberrant PhoP activation via non-cognate kinases such as YycG in the absence of PhoR. Our findings highlight the interplay between carbon catabolite repression and phosphate starvation responses, and reveal a novel mechanism by which non-cognate phosphorylation contributes to adaptive transcriptional regulation.