Structural Insights into Allosteric Regulation of GdpP: A Conformationally Dynamic Phosphodiesterase
Structural Insights into Allosteric Regulation of GdpP: A Conformationally Dynamic Phosphodiesterase
Shataer, S.; Modla, S.; Boddie, L.; Subedi, S.; Murakami, K.; Batish, M.; Parashar, V.
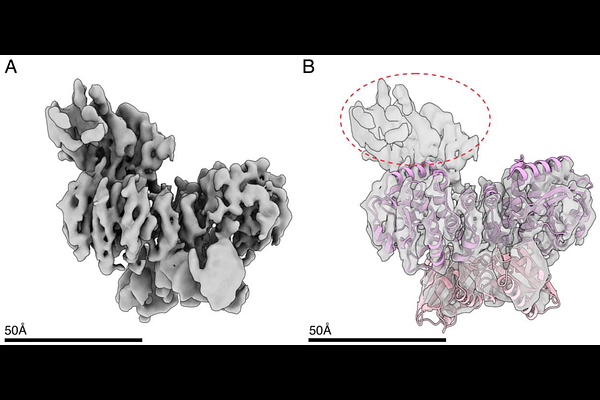
AbstractThe phosphodiesterase GdpP is a central regulator of the bacterial second messenger c-di-AMP and a key driver of antibiotic resistance in pathogenic Firmicutes. GdpP integrates environmental signals through its sensory PAS domain to control its C-terminal catalytic domain activity, but the molecular basis for this allosteric communication has remained unknown due to the lack of structural data for the complete cytosolic region. Here, we present the first Cryo-EM structures of the cytosolic region of Streptococcus mutans GdpP (SmGdpP74) in multiple conformational states, revealing a sophisticated tetrameric architecture that enables asymmetric catalytic regulation. Our structural and functional analyses demonstrate that SmGdpP74 operates through substrate-induced conformational transition, with the DHHA1 domain interface serving as the primary determinant of asymmetric catalysis. The non-canonical GGDEF domain functions as a tetrameric scaffolding hub that positions the DHH-DHHA1 catalytic domains, representing the first detailed example of a GGDEF domain repurposed to stabilize heterologous catalytic domains. We identify a flexible GGDEF-DHH linker as a critical coupling element that transmits conformational signals between domains, with the conserved KRSR motif acting as a molecular switch for heme-mediated inhibition. Additionally, the flexibility of this linker is essential for the catalytic activity of the enzyme. Based on these findings, we propose a comprehensive mechanistic model where SmGdpP74 integrates substrate availability with conformational changes for efficient hydrolysis and allosteric control of its enzymatic activity through the PAS domain. These structural insights provide a foundation for rational drug design targeting allosteric regulatory mechanisms in GdpP, potentially offering new approaches to combat antibiotic resistance in pathogenic Firmicutes.