Exploration of chemical probes and conformational flexibility of GID4 - the substrate receptor of human CTLH E3 ligase complex
Exploration of chemical probes and conformational flexibility of GID4 - the substrate receptor of human CTLH E3 ligase complex
Kotlarek, D.; Dudek, K.; Pokladek, Z.; Pastok, M. W.; Shishov, D.; Cottens, S.; Bista, M.; Krzywiecka, E.; Gorecka-Minakowska, K.; Jurczak, K.; Drmota, T.; Adamczyk, J.; Falinski, S. P.; Gajewska, D.; Klejnot, M.; Krol, A.; Cuprych-Belter, M.; Mames, I.; Mathieu, A.; Podkowka, A.; Przytulski, K.; Skowron, A. N.; Sypien, M.; Takagi, T.; Wanat, W.; Wierzbicki, I. H.; Wisniewski, J.; Szlachcic, A.; Wozniak, B.; Walczak, M. J.
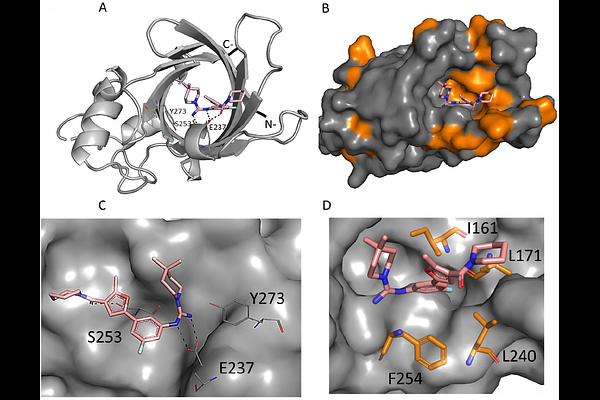
AbstractThe application of targeted protein degradation (TPD) is currently constrained by the limited availability of low-molecular-weight molecules that can recruit E3 ligases other than CRBN (Cereblon) or VHL (Von Hippel-Lindau ligase). In this study, we present the structure-based drug design (SBDD) of high-affinity ligands that engage E3 ligase GID4 (Glucose-induced degradation protein 4) in biophysical and cellular experiments. Through structural studies and molecular modeling, we identified three groups (clusters) of compounds that induce distinct conformations of GID4. We identified potential exit vectors and used the most promising ligand as a building block to prepare bifunctional degraders in the form of proteolysis-targeting chimeras (PROTACs). Although ternary complex formation was successful in vitro, degradation of BRD4 was not observed, highlighting the need for further optimization of the degraders. We also theoretically investigated the likelihood of the identified GID4 conformations participating in protein-protein interactions mediated by molecular glue mechanisms. We believe the expanded ligand diversity discovered in this study may pave the way for tuning the selectivity and efficacy of protein-protein interactions involving GID4 and its neosubstrates.