The novel RNA polymerase I transcription inhibitor PMR-116 exploits a critical therapeutic vulnerability in a broad-spectrum of high MYC malignancies.
The novel RNA polymerase I transcription inhibitor PMR-116 exploits a critical therapeutic vulnerability in a broad-spectrum of high MYC malignancies.
Ferreira, R.; Hannan, K. M.; Panov, K.; Udumanne, T.; George, A. J.; Closa, A.; Yuen, Z.; Eyras, E.; Poh, P.; Maclachlan, K.; Dwyer, M.; Barn, V.; He, J.; Kusnadi, E.; Rebello, R.; Huglo, A.; Hedwards, S.; Lawrence, M.; Risbridger, G.; Taylor, R.; Clark, A.; Farrell, G.; Pok, S.; Teoh, N.; Amarasini, M.; Norcott, P. L.; Banwell, M. G.; Manenkova, Y.; Haddach, M.; Drygin, D.; Furic, L.; Hannan, R. D.; Hein, N.
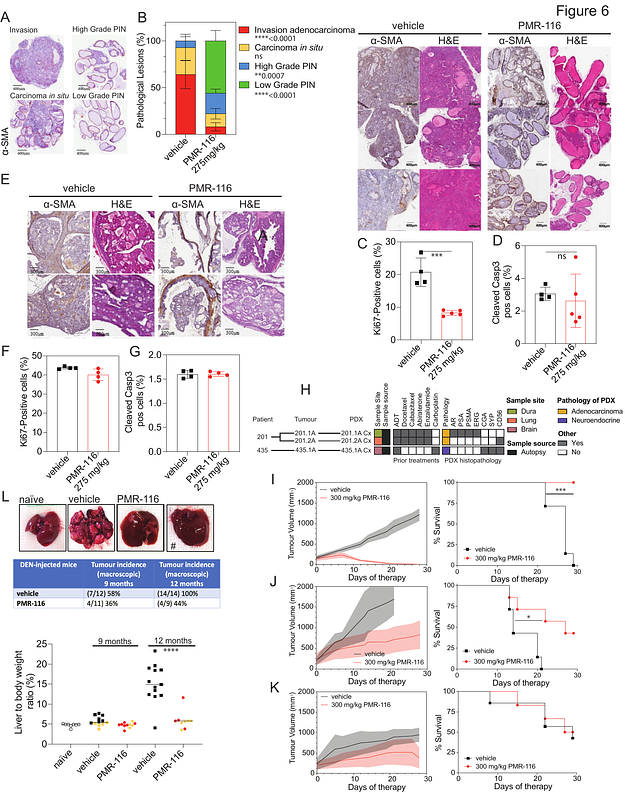
AbstractRibosome biogenesis (RiBi) is a key determinant of cell growth and proliferation and is highly elevated in cancer due to the activation by oncogenes such as MYC. First-generation RiBi inhibitor CX-5461, while demonstrating clinical potential for cancer treatment, also induces DNA damage through off-target inhibition of TOP2 and potentially other mechanisms, bringing into question RiBi as a target for cancer therapy. In this study, we test second-generation RiBi inhibitor, PMR-116. PMR-116 exhibits improved drug-like properties compared to first-generation RiBi inhibitors and has robust anti-tumour activity in the absence of global DNA damage signalling in a broad range of pre-clinical models of haematologic and solid cancers, particularly in malignancies where MYC is either the driver of disease or is elevated. Thus, our work demonstrates that RiBi is a genuine target for cancer therapy and highlights the potential to exploit a critical therapeutic vulnerability in high-MYC human cancers with dismal therapeutic outcomes.