On-cell Saturation Transfer Difference (STD) NMR on ion channels: characterizing negative allosteric modulator binding interactions of P2X7.
On-cell Saturation Transfer Difference (STD) NMR on ion channels: characterizing negative allosteric modulator binding interactions of P2X7.
Monaco, S.; Browne, J.; Wallace, M.; Angulo, J.; Stokes, L.
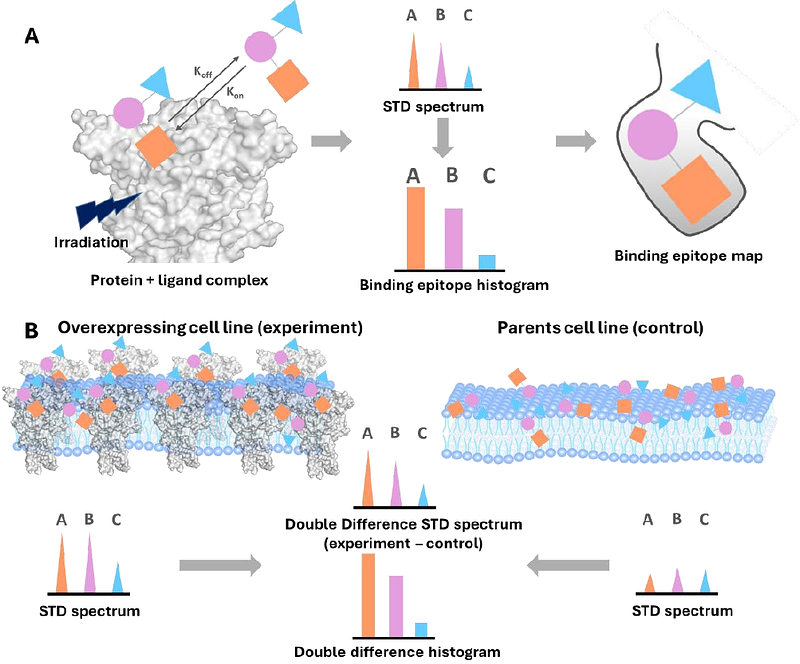
AbstractP2X7 receptors are important drug targets involved in pathologies ranging from psychiatric disorders to cancer. Being membrane embedded receptors, they are challenging for structural characterisation and at present we only have a small number of X-ray and cryoEM structures for P2X7 bound to antagonists. We demonstrate that Saturation Transfer Difference (STD) NMR on live mammalian cells (on-cell STD NMR) overexpressing P2X7 receptors allows further structural insight on the complexes of P2X7 with two potent negative allosteric modulators, namely AZ10606120 and JNJ-47465567, via the determination of the binding epitope mapping of the interactions e.g. the main region of contact between the ligand and the binding pocket. This approach, reported for the first time on membrane-embedded ion channels, in combination with molecular docking, allows us to propose the first NMR-validated 3D molecular models for two antagonists as bound to human P2X7 receptors, and to correlate the structural knowledge acquired with the pharmacology data. We highlight the transformative potential of this application to aid drug design efforts in a less resource-demanding fashion than X-ray crystallography and cryo-EM and we envisage on-cell STD NMR to fast become an asset for structure-activity-relationship studies helping knowledge-based development of efficient drugs targeting P2X7 and other ion channels/membrane-embedded proteins.