Understanding the kinetics of macrophage uptake and the metabolic fate of iron-carbohydrate complexes used for iron deficiency anemia treatment
Understanding the kinetics of macrophage uptake and the metabolic fate of iron-carbohydrate complexes used for iron deficiency anemia treatment
Eitner, S.; Kissling, V. M.; Rippl, A.; Krupnik, L.; Buergi, J.; Gaechter, S.; Hast-Sribike, K.; Gogos, A.; Bottone, D.; Bossart, J.; Riudavets Puig, R.; Digigow, R.; Alston, A. E.; Fluehmann, B.; Wick, P.; Ayala-Nunez, V.
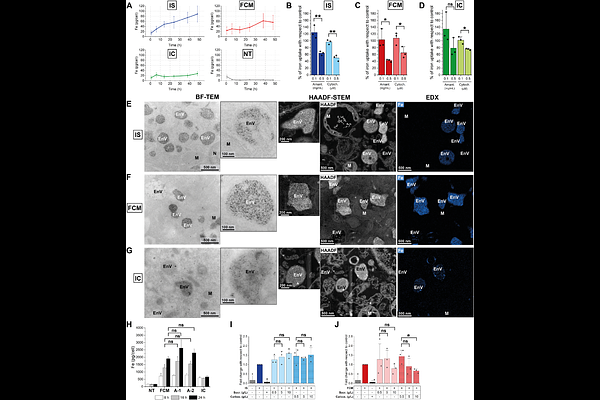
AbstractIron-carbohydrate complexes (ICCs) are widely used nanomedicines to treat iron deficiency anemia, yet their intracellular fate and the mechanisms of action underlying their differences in treatment outcomes remain poorly understood. Here, we thus performed a comprehensive dynamic characterization of two structurally distinct ICCs - iron sucrose (IS) and ferric carboxymaltose (FCM) - in primary human macrophages, key cells to the iron metabolism. By employing innovative correlative microscopy techniques, elemental analysis, and in vitro pharmacokinetic profiling, we demonstrate that the uptake, intracellular trafficking, and biodegradation of ICCs depend on their physicochemical properties. Specifically, IS is rapidly internalized and processed within endolysosomes, resulting in fast iron release and transient cytotoxicity. Conversely, FCM is sequestered in enlarged endosomes for an extended time before its biodegradation, a phenomenon we term the Hamster Effect, which leads to slower, more sustainable iron release. These results provide unprecedented insights into the metabolic fate of ICCs, enhancing our understanding of their different pharmacokinetic and pharmacodynamic profiles in vivo.