Differential Proteomic Analysis of DEN-Induced Hepatocellular Carcinoma in Male and Female Balb/c Mice Reveals Novel Gender Specific Markers
Differential Proteomic Analysis of DEN-Induced Hepatocellular Carcinoma in Male and Female Balb/c Mice Reveals Novel Gender Specific Markers
Salihah, S.; Tahir, M.; Bibi, B.; Sultan, R.; Larsen, M. R.; Mirza, M. R.; Mahmood, S.; Alam, M. R.; Gul, A.
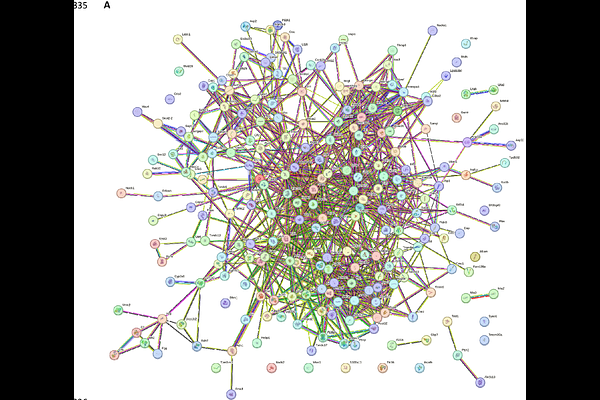
AbstractHepatocellular carcinoma is one of the leading causes of death with a higher prevalence in males compared to females due to distinct progression and pathogenesis patterns in both genders. This study aimed at creating, characterizing, and proteome profiling, a DEN-induced HCC model of male and female Balb/c mice. Proteins were extracted from characterized mouse models and subjected to mass spectrometry and subsequent bioinformatics analysis. The results revealed that the differentially enriched signaling pathways in treated female mice were mainly belonged to the constituent of cytoskeleton, and mitochondria, including metabolic and oxidoreductase activity clusters. The enriched signaling pathways in treated male mice were related to translation, ribosomal structural and binding subunits and mRNA binding, metabolic processes, glutathione transferase activity, oxidoreductase activity, and mitochondrial activity. Protein-protein interaction analysis revealed the top ten genes ranked by highest maximal clique centrality. Survival Analysis using UACLAN indicated that among the hub genes of DEN-treated female mice, only the upregulation of NDUFA8 was associated with patient survival. In DEN-treated male mice downregulation of EEF2 and upregulation of RPL7A, RPL18A, RPL27A, RPS3, RPS 11 and SERBP1 was associated with poor survival. The study indicates that the protein families associated with poor survival, ribosomal and RNA binding proteins in males, and mitochondrial complex 1 proteins in females are novel gender-specific prognostic markers and therapeutic targets for HCC.