Product-stabilized filamentation by human glutamine synthetase allosterically tunes metabolic activity
Product-stabilized filamentation by human glutamine synthetase allosterically tunes metabolic activity
Greene, E.; Muniz, R.; Yamamura, H.; Hoff, S. E.; Bajaj, P.; Lee, D. J.; Thompson, E. M.; Arada, A.; Lee, G. M.; Bonomi, M.; Kollman, J. M.; Fraser, J. S.
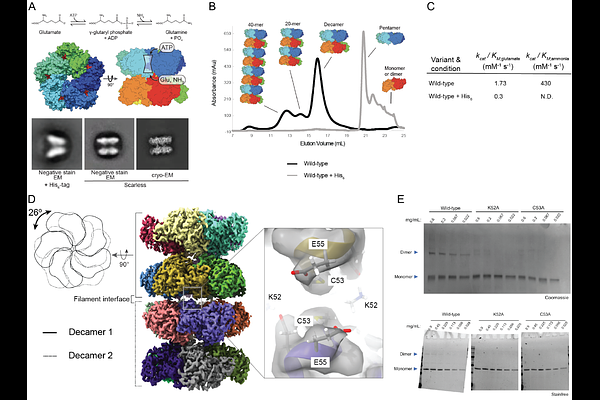
AbstractTo maintain metabolic homeostasis, enzymes must adapt to fluctuating nutrient levels through mechanisms beyond gene expression. Here, we demonstrate that human glutamine synthetase (GS) can reversibly polymerize into filaments aided by a composite binding site formed at the filament interface by the product, glutamine. Time-resolved cryo-electron microscopy (cryo-EM) confirms that glutamine binding stabilizes these filaments, which in turn exhibit reduced catalytic specificity for ammonia at physiological concentrations. This inhibition appears induced by a conformational change that remodulates the active site loop ensemble gating substrate entry. Metadynamics ensemble refinement revealed >10 [A] conformational range for the active site loop and that the loop is stabilized by transient contacts. This disorder is significant, as we show that the transient contacts which stabilize this loop in a closed conformation are essential for catalysis both in vitro and in cells. We propose that GS filament formation constitutes a negative-feedback mechanism, directly linking product concentration to the structural and functional remodeling of the enzyme.