N-Alkyl Sulfamates as a New Class of nsP2 Cysteine Protease Inhibitors with Broad Spectrum Antialphaviral Activity
N-Alkyl Sulfamates as a New Class of nsP2 Cysteine Protease Inhibitors with Broad Spectrum Antialphaviral Activity
Ghosal, A.; Sears, J. D.; Hossain, M. A.; Tse, E.; Howell, S.; Burdick, J. E.; Morales, N. L.; Martinez, S. A.; Law, I.; Streblow, Z. J.; Streblow, D. N.; Counago, R. M.; Moorman, N. J.; Heise, M. T.; Willson, T. M.
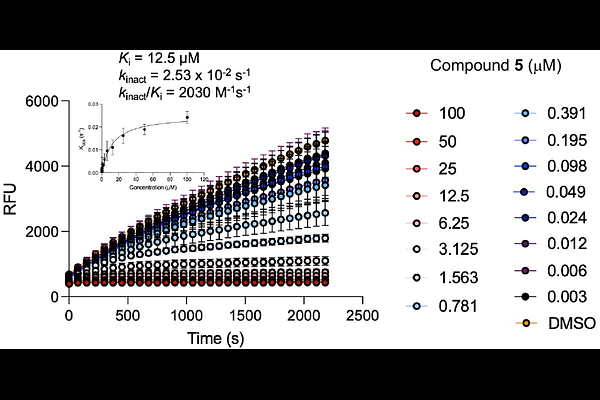
AbstractThe emergence of mosquito-borne alphaviruses that cause chronic arthritis or encephalitis underscores the urgent need for broad-spectrum antiviral therapeutics. The viral nsP2 cysteine protease, which is essential for alphavirus replication, is a promising antiviral target. Vinyl sulfone-based inhibitors, such as RA-2034, potently inhibit nsP2 protease but suffer from glutathione reactivity and species-dependent systemic clearance catalyzed by glutathione S-transferase. To address these liabilities, we explored alternative electrophilic warheads and identified reverse amide inhibitors bearing N-alkyl sulfamate warheads with improved biochemical and antiviral profiles. N-methyl sulfamate acetamide 5 emerged as a lead compound with potency against both New and Old World alphaviruses, low GSH reactivity, and high proteome-wide selectivity. Despite its promising antialphaviral activity, 5 exhibited rapid clearance due to hepatic glucuronidation. Structure-activity studies revealed modifications that improve metabolic stability while retaining antiviral activity. These findings introduce sulfamate acetamides as a new class of covalent nsP2 protease inhibitors and advance the discovery of direct acting pan-alphavirus drugs.