Hematopoietic proliferation is orchestrated by the sequential and lineage-specific activation of Cyclin D, Cyclin E and CDKN sub-modules within the G1/S network
Hematopoietic proliferation is orchestrated by the sequential and lineage-specific activation of Cyclin D, Cyclin E and CDKN sub-modules within the G1/S network
Hanel, A.; Abdelsalam, A.; Tollis, S.
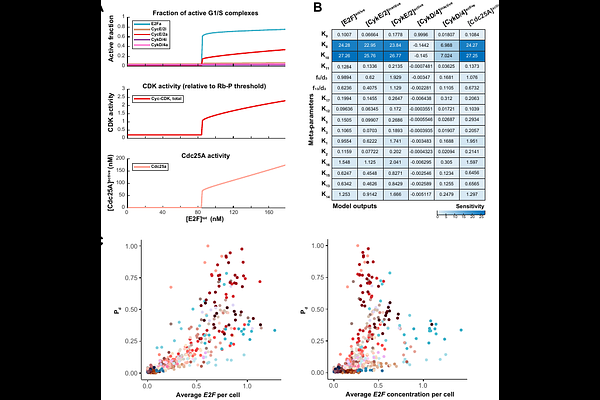
AbstractCommitment to the cell division cycle constrains other fate choices at the single cell level. Hence the molecular network controlling the G1/S transition must be coordinated with developmental phases. Healthy hematopoiesis relies on shifts in cell cycle dynamics that balance proliferation and differentiation of hematopoietic stem cells (HSCs) and lineage progenitors, offering an ideal model system to study the coordination of cell division with development. The G1/S network orchestrates this process by regulating the activites of the master G1/S transcription factors (E2F), and cyclin dependent kinases whose activities drive the progression to S phase. By using single cell transcriptomics profiles of human bone marrow cells and mathematical modeling, we demonstrate that variations in the expression of cyclin D- and cyclin E-centered sub-modules of the G1/S network carve out distinct trajectories from G1 to S, explaining the distinct proliferation properties of hematopoietic cell types evolving in the same microenvironment, and biasing cell fate decisions towards certain lineages. We map 68 hematopoietic cell types to specific model parameters, and identify their individual route through G1/S, using our model. This improved mechanistic understanding of the G1/S transition across cell types could enhance the design of more nuanced pharmacological strategies, enabling more personalized treatment recommendations for current cell cycle-targeted cancer therapies.