Phosphorylation-inducing chimera rewires oncogenic kinase to trigger apoptosis
Phosphorylation-inducing chimera rewires oncogenic kinase to trigger apoptosis
Merz, M. M.; Shoba, V. M.; Pergu, R.; Severance, Z. C.; Munkanatta Godage, D. N. P.; Deb, A.; Kwok, H. S.; Singh, P.; Singh, S.; Allen, J. B.; Tian, W.; Gosavi, P. M.; Chaudhary, S. K.; Anokhina, V.; Weisberg, E. L.; Payne, N. C.; He, Y.; Osadchey, R.; Shekhar, M.; Mazitschek, R.; Rees, M. G.; Roth, J. A.; Cui, Q.; Griffin, J. D.; Liau, B. B.; Choudhary, A.
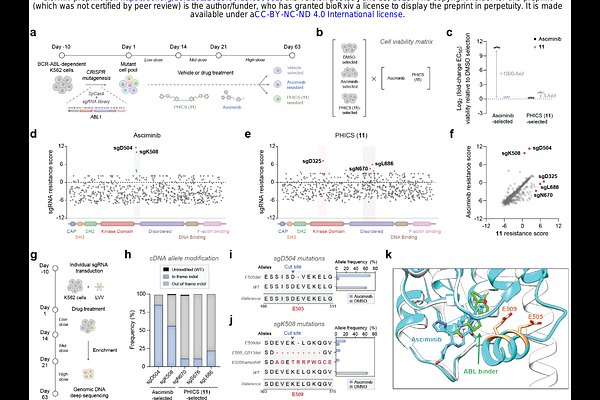
AbstractHyperactive enzymes drive the pathology of several diseases, and classically, \'\'occupancy-driven\'\' drugs (e.g., active site or allosteric inhibitors) are used to target these enzymes. However, the stoichiometric nature of such inhibitors and the emergence of resistance highlight the need for new modalities. Here, we report a Phosphorylation-Inducing Chimeric Small molecule (PHICS) that rewires the hyperactivity of an oncogenic kinase, BCR-ABL, to phosphorylate its active site residue. Molecular dynamics simulations suggest this phosphorylation inhibits BCR-ABL by inducing electrostatic rearrangements of its active site. This \'\'event-driven\'\' mechanism selectively induces apoptosis of BCR-ABL-dependent cancer cells at substoichiometric concentrations (vs. stoichiometric concentrations of occupancy-driven drugs). Furthermore, PHICS is effective on other oncogenic ABL fusions or clinically observed resistance mutations, including to occupancy-driven drugs with the same binding site as PHICS, pointing to the orthogonality of their resistance mechanisms. These studies lay the foundation for electric-field and \'\'event-driven\'\' modalities to control hyperactive enzymes with orthogonal resistance mechanisms to occupancy-driven modalities.