Inter-Spheroid Proximity and Matrix Remodeling Determine CAF-Mediated Cancer Cell Invasion
Inter-Spheroid Proximity and Matrix Remodeling Determine CAF-Mediated Cancer Cell Invasion
Mehta, P. P.; Bordoloi, A. D.; Ravensbergen, C.; David, K.; Liefers, G.-J.; Mesker, W.; ten Dijke, P. P.; Boukany, P.
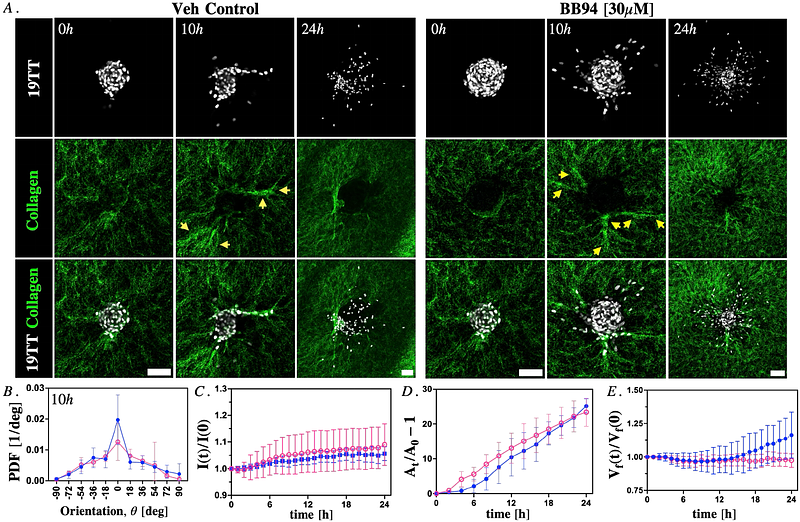
AbstractBreast cancer is the most commonly diagnosed malignancy worldwide, with molecular subtypes following distinct clinical trajectories. While Luminal A breast cancers are typically indolent, a subset enriched in -smooth muscle actin (-SMA)-positive cancer-associated fibroblasts (CAFs) exhibits aggressive behavior, facilitating tumor invasion. However, the biophysical mechanisms by which CAFs drive invasion and extracellular matrix (ECM) remodeling remain unclear. Furthermore, the temporal and spatial dynamics of CAF interactions with the collagen matrix and cancer cell spheroids remain unknown, raising the question of whether these processes follow a deterministic sequence or occur stochastically. To address this, we conduct histological analysis of Luminal A tumors, revealing variation in CAF, cancer cell, and ECM organization at tumor boundaries. To assess the impact of CAF on cancer cell invasion, we use a 3D in-vitro model co-embedding 19TT breast CAF and MCF7 luminal breast cancer spheroids within a three-dimensional (3D) collagen-I hydrogel and perform time-lapse imaging. We demonstrate that inter-spheroid distance critically determines 19TT CAF-induced MCF7 spheroid behavior. Furthermore, we show that CAF-mediated collagen matrix remodeling and degradation precedes MCF7 spheroid disruption and is critical in promoting cancer cell spheroid expansion and cell dissemination. While broad-spectrum matrix metalloproteinase inhibition suppresses CAF-driven collagen degradation and MCF7 spheroid expansion, it does not prevent ECM remodeling, CAF migration, or single-cell dissemination of cancer cell spheroids. Furthermore, a complementary heterospheroid model reveals similar ECM remodeling and invasion dynamics despite altered cellular arrangement of cancer cells and CAFs. These findings enhance our understanding of the relationship between CAF activity and collagen matrix remodeling processes that promote cancer cell invasion, providing insights into the potential therapeutic benefits of targeting CAFs in breast cancer treatment.