Cardiac Adaptations in the Cave Nectar Bat Eonycteris spelaea: Insights into Metabolic Resilience and Stress Response
Cardiac Adaptations in the Cave Nectar Bat Eonycteris spelaea: Insights into Metabolic Resilience and Stress Response
Yu, F.; Gamage, A. M.; Kp, M. M. J.; Foo, R.; Lin, Y.-H.; Wang, L.; Pua, C. J.; Chan, W.; Crespo-Avilan, G. E.; Pena, E. M.; Hong, L. Z.; Iyer, A.; Ghosh, S.; Liehn, E. A.; Kovalik, J.-P.; Wang, L.-F.; Ramachandra, C. J.; Hausenloy, D. J.
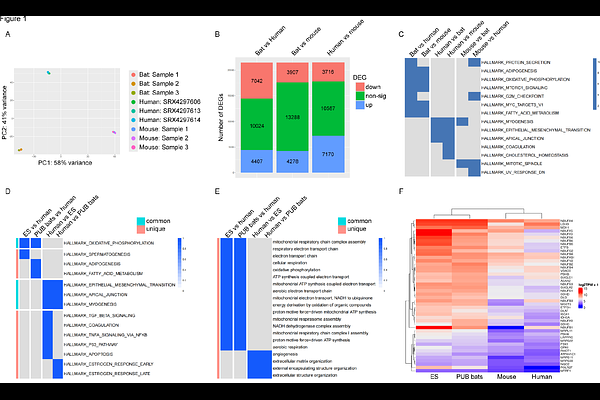
AbstractAims: Bats are unique mammals with remarkable adaptations, including powered flight, which demands significant energy expenditure. While previous studies have documented basic structural characteristics of bat hearts, a comprehensive understanding of their response to physiological stress remains unexplored. This study investigates the cardiac adaptations of the cave nectar bat Eonycteris spelaea to elucidate the mechanisms underlying their unique physiological capabilities. Methods and Results: We performed RNA sequencing to analyse the cardiac gene expression profile of E. spelaea and 6 other bat species in comparison to mouse and human hearts, revealing enriched transcriptomic signatures related to oxidative phosphorylation and fatty acid metabolism across multiple bat species. Metabolomic profiling compared acylcarnitine metabolites and tricarboxylic acid (TCA) cycle intermediates between bat and mouse hearts, indicating a distinct acylcarnitine profile and increased levels of TCA cycle intermediates in bats, suggesting enhanced metabolic capacity. Structural adaptations were assessed through anatomical and histological analyses on cardiac tissues, showing thicker left ventricular walls and increased vascular density in bats without pathological hypertrophy. Functional characteristics were evaluated using dobutamine stress echocardiography, demonstrating superior cardiac reserve in bats with significant increases in ejection fraction, stroke volume, and cardiac output under stress conditions. Additionally, isolated cardiomyocytes were treated with Angiotensin II (Ang II) to assess stress responses. Bat cardiomyocytes displayed resistance to Ang II-induced hypertrophy and mitochondrial dysfunction compared to mice, further highlighting their resilience to stress-induced damage. Conclusion: The unique adaptations observed in bat hearts, including enhanced metabolic pathways, structural remodelling, and cellular resilience contribute to their ability to meet the high energy demands of powered flight while maintain cardiac function under stress. These insights into bat cardiac physiology provide valuable information on cardioprotective mechanisms that could be applicable to other species.