Heterogeneous NF-κB activation and enhancer features shape transcription in Drosophila immunity
Heterogeneous NF-κB activation and enhancer features shape transcription in Drosophila immunity
Nawar, N.; Rits, E.; Cohen, L.; Wunderlich, Z.
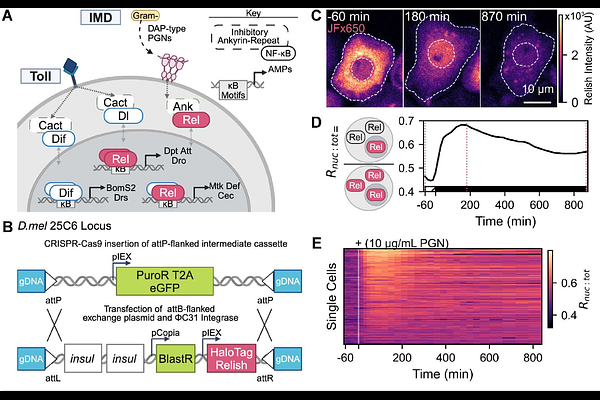
AbstractConserved NF-{kappa}B signaling pathways shape immune responses in animals. In mammals, NF-{kappa}B activation patterns and downstream transcription vary with stimulus, cell type, and stochastic differences among identically treated cells. Whether animals without adaptive immunity exhibit similar heterogeneity or rely on distinct immune strategies remains unknown. We engineered Drosophila melanogaster S2* reporter cells as an immune-responsive model to monitor the dynamics of an NF-{kappa}B transcription factor, Relish, and downstream transcription in single, living cells. Following immune stimulation, Relish exhibits diverse nuclear localization dynamics that fall into distinct categories, with both the fraction of responsive cells and their activation speed rising with stimulus dose. Pre-stimulus features, including Relish nuclear fraction, predict a cell\'s responsiveness to stimulation. Simultaneous measurement of Relish and downstream transcription revealed that the probability of transcriptional bursts from immune-responsive enhancers correlates with Relish nuclear fraction. The number of NF-{kappa}B binding sites tunes transcriptional activity among immune enhancers. Our study uncovers heterogeneity in NF-{kappa}B activation and target gene expression within Drosophila, illustrating how dynamic NF-{kappa}B behavior and enhancer architecture tune gene regulation.